相關(guān)文章
Related Articles簡(jiǎn)要描述:HyClone Characterized FBS, AUS Origin, 1000 mL, Heat-inactivated;澳洲特級(jí)胎牛血清
 產(chǎn)品型號(hào):型號(hào)齊全
產(chǎn)品型號(hào):型號(hào)齊全 廠商性質(zhì):代理商
廠商性質(zhì):代理商 更新時(shí)間:2024-12-17
更新時(shí)間:2024-12-17 訪 問(wèn) 量:741
訪 問(wèn) 量:741澳洲特級(jí)胎牛血清
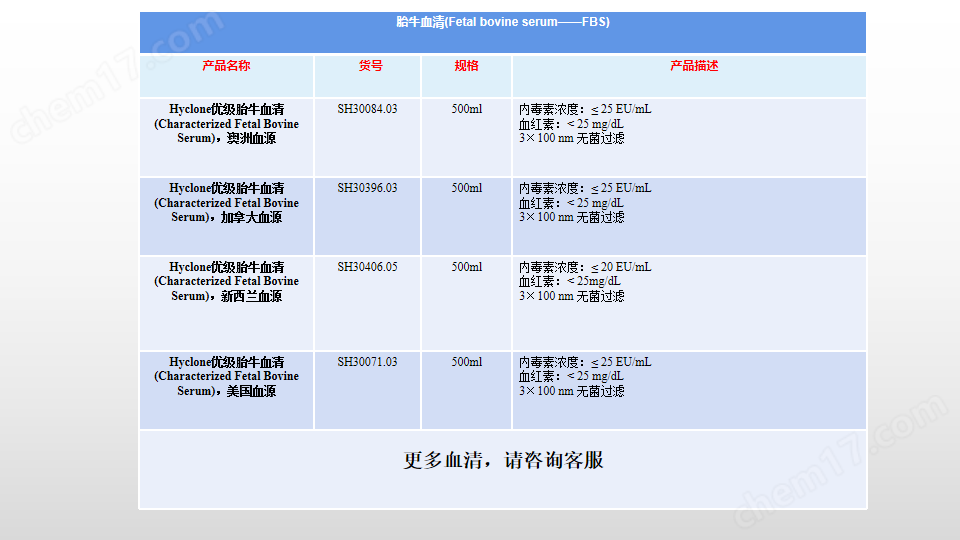
產(chǎn)品名稱: HyClone Characterized FBS, AUS Origin, 1000 mL, Heat-inactivated;澳洲特級(jí)胎牛血清
產(chǎn)品貨號(hào):SH30084.04HI;
處理方式:3×100 nm濾膜過(guò)濾;
地區(qū)來(lái)源:澳大利亞;
物種來(lái)源:;
內(nèi)毒素水平:內(nèi)毒素水平:≤ 25 EU/mL;
血紅素水平:< 25 mg/dL
熱滅活:滅活;
射線處理:未處理;
保存條件:≤-10℃;
運(yùn)輸方式:干冰運(yùn)輸;
詳細(xì)介紹:This premium HyCloneTM Fetal Bovine Australian-origin Serum is well-characterized, triple 100 nm filtered, virus-tested to 9 CFR standard, and traceability-certified (ISIA, Oritain).
Australian origin: benefit from Australian husbandry methods and certified traceability.
Characterized: assayed for a variety of critical components and biochemical characteristics.
Endotoxin-tested: certificate of analysis confirms ≤ 25 EU/mL endotoxin.
Triple-filtered: serial 100 nm pore size-rated filtering for reliable microbial depletion.
Post-filtration treatments: γ-irradiated and heat-inactivated FBS options also available.
Virus-tested: broad virus panel testing in accordance with 9 CFR 113.53.
Fetal bovine serum (FBS) is a nutrient-rich cell culture supplement that is high in growth factors and low in antibodies, compared to non-fetal sera.
Why choose HyCloneTM Australian Origin FBS?
HyClone Australian Origin serum is sourced from Australian abattoirs approved by the U.S. Department of Agriculture (USDA) or Australian Quarantine and Inspection Service (AQIS). The Australian methods of animal husbandry exhibit excellent animal nutrition and healthcare. Australia is an isolated continent, thus making animal disease and control management easier than in most areas of the world.
Characterized FBS for cell culture
HyClone Characterized FBS is an excellent alternative to more expensive defined sera, and meets the requirements of most discriminating FBS users. The final serum is assayed for important proteins, hormones, growth factors, metabolites, and nutrients that support healthy cell cultures. Assay results are included in the certificate of analysis and biochemical assay list. Additional testing, including EMEA-conforming testing, is also available.
Have Confidence in Security of Supply with Secondary Sourcing
Fetal Bovine Serum from a single origin may be limited, so it’s important to qualify FBS products from other countries to increase your security of supply. HyClone Characterized FBS from United States (US), New Zealand (NZ) and Australia (AUS) have been evaluated and tested to show equivalent performance. Read our full study.
Bacterial endotoxin-tested
Endotoxin contamination is a significant concern in research and manufacturing, especially when producing cell culture-derived products for use in humans or animals. The presence of endotoxin in cell cultures can also have adverse and unpredictable effects on cell growth, function and phenotype that can compromise research results. Our patented closed-system blood collection method and rigorous quality control testing ensure that HyClone Characterized FBS meets industry standards of ≤ 25 EU/mL endotoxin. The final product also contains ≤ 25 mg/dL hemoglobin, which is an indicator of appropriate handling during collection and transport.
Virus-tested and quality-assured for reduced contamination risk
HyClone Characterized FBS is triple-filtered through serial 100 nm (0.10 μm) pore size-rated filters, which has become an industry standard for cell culture grade FBS. Before dispensing, each lot of serum is pooled using true pool technology to minimize variability from bottle to bottle.
The final serum is virus-tested according to 9 CFR 113.53 to demonstrate that there are no detectable levels of specified viruses, pathogenic agents, and hemadsorbing agents. Robust sterility testing further confirms that the final product contains no detectable growth of bacteria or fungi (current USP) and no detectable mycoplasma.
Post-filtration treatments for added process security
Gamma-irradiated and heat-inactivated FBS options are available to further reduce the risk of adventitious agents, particularly when manufacturing products for use in humans or animals. The irradiation process is validated to deliver a minimum dose of 25 kGy.
Regulatory and supply chain security
For decades, Cytiva's HyClone™ premium bovine serum products have exceeded industry standards for quality, purity, and regulatory compliance. HyClone™ serum processes are traceability traceability-certified by the International Serum Industry Association (ISIA) and, . Iin addition, Cytiva has chosen to be an early adopter of scientific traceability by Oritain to prove the country of origin of their premium bovine serum products from the United States, Australia and New Zealand. Premium bovine serum samples from different regions have been analyzed and Oritain has created a database of the unique ‘Origin Fingerprint’ that is exclusive to each country of origin.